Table of Contents
Introduction
Dental composites, or resin-based composites,is a cement made of synthetic resins1. It is a mixture of plastic (acrylic) resin that’s reinforced with a powdered glass filler
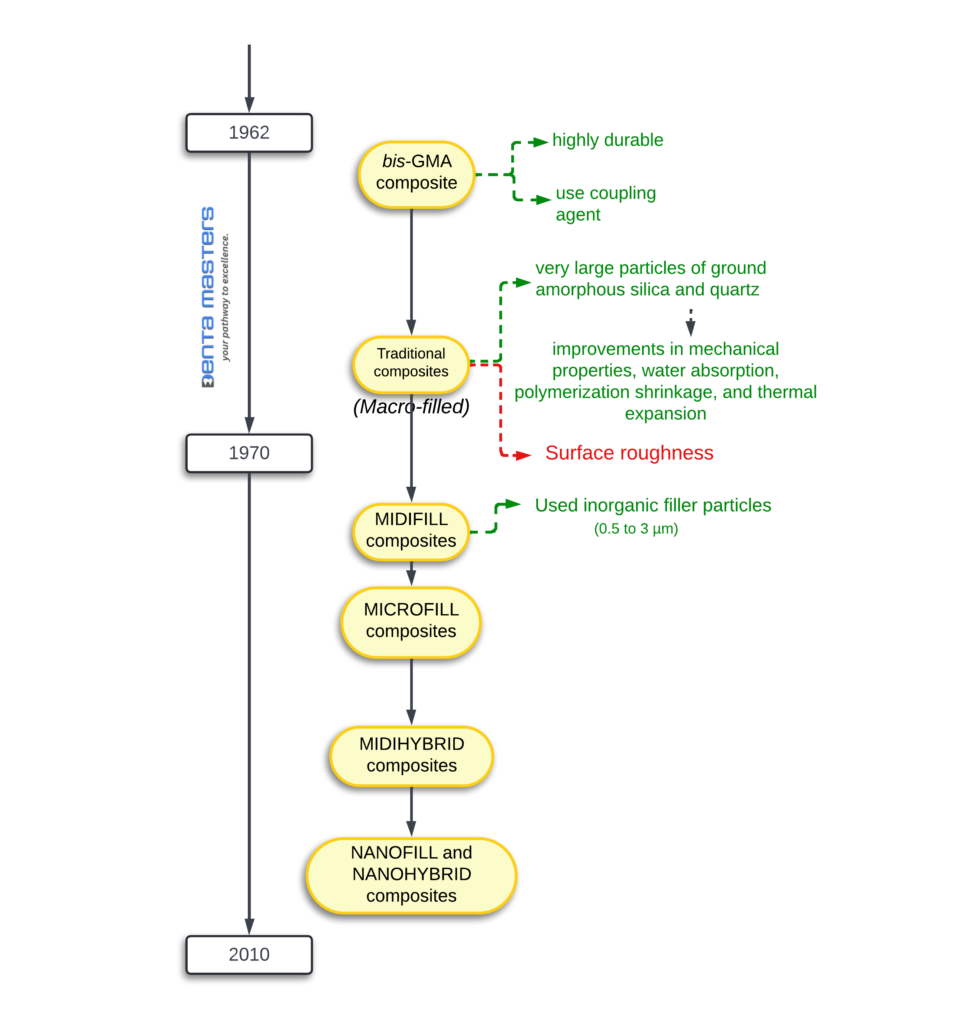
Evolution of composite
1970s: First commercially successful restorative composite resins (Concise [3M], Adaptic [Dentsply Sirona]) introduced. Macrofills with large filler particles (0–5 μm). Irregular-edged particles made polishing difficult despite strength.
1980s: Microfill composites debuted (Durafill VS [Kulzer], Renamel [Cosmedent]). Excellent polishability and aesthetics, but lacked strength for occlusal load-bearing areas. Exception: heliomolar (Ivoclar Vivadent), a microfill for posterior teeth.
1990s: Hybrids and layering of composites became popular. Early hybrids lacked polishability. Later products (e.g., Esthet-X [Dentsply Sirona]) were stronger but had inferior aesthetics compared to modern composites.
2000s: New formulations with improved aesthetics. Translucent and opaque shades allowed mimicking tooth structure. Nanofilled composites introduced (e.g., Tetric EvoCeram [Ivoclar Vivadent], Filtek Supreme Plus [3M]). Nanoparticles (5–75 nm) and nanoclusters (5–20 nm) provided smoother finishes, stronger restorations, and better shine.
2010s: Bulk-fill composites gained acceptance. Promoted less polymerization shrinkage and higher depth of cure (up to 4.0 mm). Early flowable bulk-fill (SureFil SDR flow [Dentsply Sirona]) limited to base use. Newer flowable bulk-fill agents (e.g., Tetric EvoCeram Bulk Fill [Ivoclar Vivadent], Estelite Bulk Fill Flow [Tokuyama Dental America]) require no capping layer, offering higher strength and aesthetics. Translucency varies, which can be advantageous or disadvantageous depending on the case.

Dental Composites: Composition and Key Components
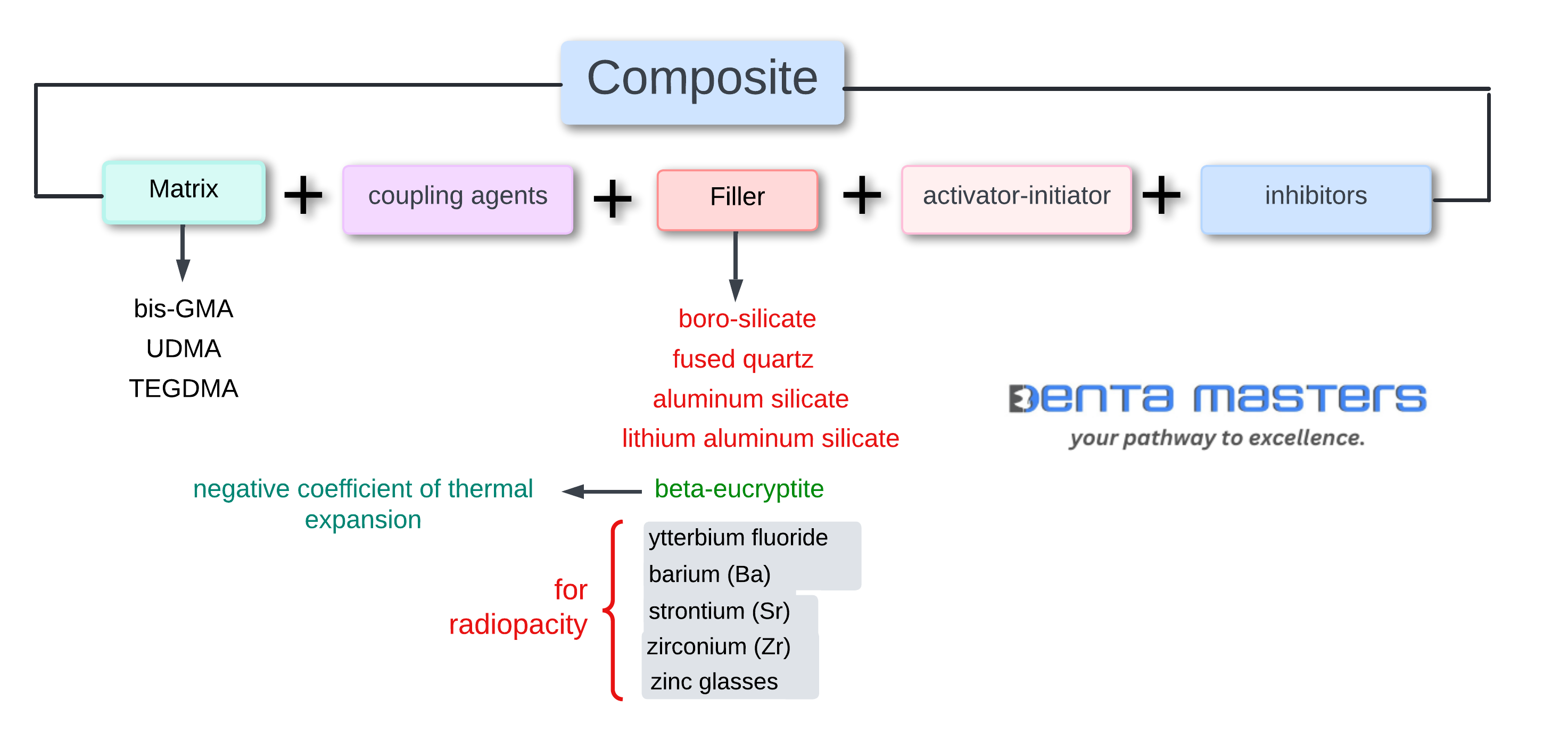
Dental composites are versatile materials used for restorative procedures, and their performance depends on their carefully engineered composition. Here’s a breakdown of the major components and additives that make up dental composites:
1. Resin Matrix
- Role: Forms the base structure of the composite.
- Composition: A highly cross-linked polymeric material, typically made of dimethacrylate monomers (e.g., Bis-GMA, UDMA, TEGDMA).
- Function: Provides strength, durability, and moldability.
2. Filler Particles
- Role: Reinforce the resin matrix and improve physical properties.
- Composition: Made of glass, silica, crystalline, or metal oxide particles.
- Function: Enhance strength, wear resistance, and polishability.
3. Coupling Agents
- Role: Bond the resin matrix and filler particles together.
- Composition: typically silane compounds.
- Function: Improve adhesion between the organic (resin) and inorganic (filler) phases, enhancing overall material performance.
Additional Components
4. Activator-Initiator System
- Role: Trigger polymerization to harden the composite.
- Types:
- Light-Cure Systems: Use a photoinitiator (e.g., camphorquinone) activated by a curing light.
- Chemically Activated Systems: Use a chemical initiator (e.g., benzoyl peroxide) for self-curing.
- Function: Convert the soft, moldable resin paste into a hard, durable restoration.
5. Pigments
- Role: Match the color of natural tooth structure.
- Function: Provide aesthetic appeal by mimicking the shade and translucency of teeth.
6. Ultraviolet (UV) Absorbers
- Role: Protect the composite from discoloration.
- Function: Improve color stability by blocking UV light that can degrade the material over time.
7. Inhibitors
- Role: Control the polymerization process.
- Function:
- Extend storage life by preventing premature curing.
- Provide increased working time for chemically activated resins by slowing polymerization until needed.
Dental Composite Resin Matrix: Key Monomers and Their Properties
The resin matrix in dental composites is primarily based on dimethacrylate monomers, which form the backbone of the material. These monomers are carefully selected and blended to achieve the desired properties, such as strength, durability, and ease of use. Here’s a detailed look at the key monomers and their roles:
1. Bis-GMA (Bisphenol A Glycidyl Methacrylate)
- Nature: Known as the “Bowen monomer”, it is hydrophobic and forms highly cross-linked, strong, and rigid polymer structures.
- Properties:
- High molecular weight (M = 512 g/mol).
- High viscosity, making it difficult to blend and manipulate.
- Refractive index: ~1.55.
2. UDMA (Urethane Dimethacrylate)
- Nature: A flexible monomer that also forms strong, durable polymer structures.
- Properties:
- Lower molecular weight (M = 470 g/mol) compared to Bis-GMA.
- More flexible than Bis-GMA, providing better stress distribution.
- High viscosity, similar to Bis-GMA.
3. TEGDMA (Triethylene Glycol Dimethacrylate)
- Nature: A low-molecular-weight, highly fluid monomer used as a diluent.
- Properties:
- Reduces viscosity when blended with Bis-GMA or UDMA.
- Example: A blend of 75% Bis-GMA + 25% TEGDMA has a viscosity of 4300 centipoise, while a 50% Bis-GMA + 50% TEGDMA blend has a viscosity of 200 centipoise (similar to thin syrup).
- Refractive index: ~1.46.
Challenges with Monomer Blends
1. Polymerization Shrinkage:
- Smaller monomers like TEGDMA undergo greater shrinkage during polymerization, increasing the risk of marginal gaps and leakage.
- This partially offsets the advantage of using larger monomers like Bis-GMA.
2. Refractive Index Matching:
- Bis-GMA and TEGDMA have refractive indices of ~1.55 and ~1.46, respectively.
- A 50:50 blend by weight yields a refractive index of ~1.50, which matches well with the refractive index of most filler particles (e.g., glass or quartz at ~1.50). This ensures adequate translucency and aesthetic appeal.
Filler Particles in Dental Composites: Types and Functions
Filler particles are a critical component of dental composites, providing strength, reinforcement, and reduced curing shrinkage and thermal expansion. They typically make up 30% to 70% by volume or 50% to 85% by weight of the composite. Here’s a detailed look at the types of fillers and their roles:
Types of Filler Particles
1. Glass Fillers
- Soft Glass: Often used for its ease of processing and ability to be ground into fine particles.
- Borosilicate Hard Glass: Provides strength and durability.
2. Quartz Fillers
- Fused Quartz: Chemically inert and highly durable.
- Drawbacks:
- Very hard, making it abrasive to opposing teeth and restorations.
- Difficult to grind into fine particles and polish.
3. Silicate Fillers
- Aluminum Silicate: Commonly used for its strength and compatibility with the resin matrix.
- Lithium Aluminum Silicate (Beta-Eucryptite): Unique for its negative coefficient of thermal expansion, which helps reduce stress during temperature changes.
4. Radiopaque Fillers
- Ytterbium Fluoride: Provides radiopacity for better visibility on X-rays.
- Heavy metal glasses:
- Barium (Ba), strontium (Sr), zirconium (Zr), and zinc glasses: These fillers contain heavy metal atoms that impart radiopacity, making restorations easier to detect radiographically.
Functions of Filler Particles
1. Reinforcement of Physical and Mechanical Properties
Fillers significantly enhance the following properties, which are critical for clinical performance:
- Compressive Strength: Ability to withstand chewing forces.
- Tensile Strength: Resistance to breaking under tension.
- Modulus of Elasticity (Stiffness/Rigidity): Provides structural integrity.
- Toughness: ability to absorb energy without fracturing.
As the filler volume fraction approaches ~70%, the composite’s abrasion and fracture resistance reach levels comparable to natural tooth tissue.
2. Reduction of Polymerization Shrinkage
- How It Works: Fillers reduce curing shrinkage in proportion to their volume fraction.
- Benefit: minimizes marginal gaps and leakage, improving the longevity of the restoration.
3. Reduction in Thermal Expansion and Contraction
- How It Works: Glass and ceramic fillers have lower thermal expansion coefficients than polymers. Increasing filler content reduces the composite’s overall thermal expansion, bringing it closer to that of natural tooth tissue.
- Benefit: Reduces interfacial stress caused by temperature changes (e.g., hot and cold foods), preventing microleakage and restoration failure.
4. Control of Workability and Viscosity
- How It Works: Adding fillers to fluid monomers creates a paste. Higher filler loading increases thickness, while filler size and shape influence consistency.
- Benefit: affects clinical handling properties, such as:
- Ease of mixing and sculpting.
- Tackiness and shape retention.
- Minimizing slumping during placement.
5. Decreased Water Sorption
- How It Works: Increased filler loading reduces water absorption by the resin matrix.
- Benefit: Prevents softening of the resin, reducing the risk of abrasive wear and staining.
6. Imparting Radiopacity
- Why It’s Important: Resins are naturally radiolucent, making it difficult to detect issues like leaking margins, secondary caries, or proximal wear on X-rays.
- How It Works: Radiopacity is achieved by adding glass fillers containing heavy metal atoms (e.g., barium, strontium, zirconium).
- Benefit: Ensures restorations are visible on radiographs, aiding in diagnosis and monitoring.
Challenges with Glass Fillers
While glass fillers containing heavy metals (e.g., barium, strontium, zirconium) are widely used to provide radiopacity in dental composites, they come with certain limitations. Here’s a closer look at the issues and their implications:
1. Lack of Chemical Inertness
- Problem: Unlike quartz and amorphous silica, glass fillers are not completely inert.
- Leaching: Over time, these fillers can be slowly leached and weakened when exposed to:
- Acidic liquids (e.g., citrus juices, sodas).
- High pH solutions (e.g., alkaline oral fluids).
- Acidulated phosphate fluoride (APF) solutions or gels are used for caries prevention.
2. Increased Susceptibility to Abrasive Wear
- Problem: As glass fillers degrade, the composite becomes more prone to abrasive wear.
- Implication: This reduces the functional lifetime of the restoration compared to composites reinforced with silica or quartz.
Why This Matters
- Durability: Glass-filled composites may not last as long as silica-reinforced resins, especially in patients with high acidic diets or those using fluoride treatments.
- Clinical Performance: Degradation of fillers can lead to surface roughness, staining, and loss of material, compromising the restoration’s aesthetics and function.
Solutions and Alternatives
- Silica or Quartz Fillers:
- More chemically inert and resistant to acidic environments.
- Ideal for patients with high caries risk or acidic diets.
- Hybrid Composites:
- Combine glass and silica fillers to balance radiopacity and durability.
- Improved filler coatings:
- Use advanced coatings to protect glass fillers from acidic degradation.
- Patient Education:
- Advise patients to limit acidic foods and beverages.
- Recommend neutral fluoride products instead of acidulated phosphate fluoride.
Advantages of Small Filler Particles
- If particle size is uniform, there will always be spaces (voids) among the particles, regardless of how tightly they are packed. This is illustrated by the example of void spaces in a packed system (like a box filled with spheres of the same size).
- However, if smaller particles are inserted among the larger ones (e.g., Bis-GMA, UDMA, TEGDMA), the void space can be reduced, leading to a denser, more compact material structure.
- Another advantage of using smaller particles is the improvement in aesthetic properties (appearance) and surface smoothness (polishability), which enhances both visual appeal and tactile sensation (smoothness when touched by the tongue).
- Particles larger than the wavelength of visible light (approximately 400-700 nm) can cause light scattering.
- Light scattering increases the opacity of the material, making it less translucent and causing a visibly rough surface texture when these larger particles are exposed at the surface.
- A roughened surface not only affects the aesthetic appearance but also tends to accumulate stains and plaque over time, compromising both the esthetic quality and oral hygiene of the restoration.
Coupling Agents
A coupling agent is a critical component in dental composites that helps bond the fill particles to the resin matrix. Without a coupling agent, the filler particles and resin matrix would not adhere properly, leading to a weaker material that is more prone to wear and failure.
What Is a Coupling Agent?
Dual-Functionality:
- A coupling agent is a difunctional surface-active compound, meaning it has two key roles:
- It adheres to the surface of filler particles.
- It coreacts with the monomer (the liquid component) that forms the resin matrix.
- This dual functionality creates a strong bond between the filler particles and the resin, ensuring the composite material functions as a single, cohesive unit.
- A coupling agent is a difunctional surface-active compound, meaning it has two key roles:
Most Common Type:
- Silane Coupling Agents:
- The most widely used coupling agents in dentistry are organic silicon compounds, known as silane coupling agents.
- These agents are particularly effective because they form strong chemical bonds with both the filler particles and the resin matrix.
How Does a Silane Coupling Agent Work?
Surface Treatment of Fillers:
- During the manufacturing process, the filler particles are treated with the silane coupling agent.
- The methoxy groups (-OCH₃) in the silane molecule undergo hydrolysis (a chemical reaction with water) in the presence of an acid or base catalyst. This reaction generates hydroxyl groups (-OH).
Forming the Interfacial Bridge:
- The hydroxyl groups on the silane molecule then react with the surface of the filler particles, creating a strong bond.
- At the same time, the other end of the silane molecule reacts with the resin monomer, forming a covalent bond.
- This creates an interfacial bridge that strongly binds the filler particles to the resin matrix.
Why Are Coupling Agents Important?
Enhanced Mechanical Properties:
- The coupling agent significantly improves the strength, durability, and wear resistance of the composite material.
- Without a coupling agent, the filler particles could separate from the resin matrix under stress, leading to failure of the restoration.
Prevents Plucking:
- “Plucking” refers to the phenomenon where filler particles are dislodged from the resin matrix due to weak bonding. This can lead to surface roughness, wear, and eventual failure of the restoration.
- The coupling agent minimizes plucking by ensuring a strong, stable bond between the filler and resin.
Longevity of the Restoration:
- By improving the bond between the filler and resin, coupling agents contribute to the long-term performance of dental composites, making them more reliable for use in restorative dentistry.
Initiators and Accelerators in Dental Composites
In dental composites, initiators and accelerators are essential components that trigger and speed up the polymerization process (curing) of the material. This process transforms the soft, moldable composite into a hard, durable restoration. There are two main methods of activation: light activation and chemical activation.
Light Activation: How It Works
Blue Light Activation:
- Dental composites are typically cured using blue light with a peak wavelength of about 465 nm.
- This light is absorbed by a photosensitizer, usually camphorquinone, which is added to the monomer mixture during manufacturing in concentrations ranging from 0.1% to 1.0%.
Role of Camphorquinone:
- When exposed to blue light, camphorquinone absorbs the energy and generates free radicals.
- These free radicals are highly reactive and initiate the polymerization process by attacking the carbon double bonds (C=C) in the methacrylate monomers.
Acceleration by Organic Amine:
- The reaction is accelerated by the presence of an organic amine, which acts as a co-initiator.
- The amine donates electrons to the camphorquinone, enhancing the generation of free radicals and speeding up the curing process.
Stability in the Dark:
- The camphorquinone and amine remain stable at room temperature as long as the composite is not exposed to light. This ensures the material doesn’t cure prematurely during storage or handling.
Chemical Activation: How It Works
Two-Paste System:
- Chemically activated composites come in two separate pastes:
- Universal Paste: Contains an organic peroxide (e.g., benzoyl peroxide).
- Catalyst Paste: Contains an organic amine.
- When the two pastes are mixed, the amine reacts with the peroxide to produce free radicals.
- Chemically activated composites come in two separate pastes:
Free Radical Generation:
- The free radicals attack the carbon double bonds (C=C) in the methacrylate monomers, initiating polymerization.
- This reaction proceeds rapidly at room temperature, making chemically activated composites ideal for situations where light curing is not feasible.
| Mechanism | Proposed By | Key Findings |
|---|---|---|
| Peripheral Vasodilation | Sharpey-Schafer (1953) | Cough → ↓BP → Syncope |
| Reduced Cardiac Output | Gastaut et al. (1966) | ↓Venous return → Cerebral hypoperfusion |
| Increased ICP ("Concussion") | Kerr et al. (1961) | Cough → ↑ICP → Brainstem compression |
| Cerebral Circulatory Arrest | Mattle et al. (1995) | TCD shows MCA flow cessation during cough |
| Carotid Blood Flow Reduction | Desser et al. (1973) | ↓Carotid velocity (18–62%) during cough |
Why are initiators and accelerators important?
- Control Over Curing:
- Light activation allows dentists to control when and where the composite cures, providing flexibility during placement.
- Chemical activation is useful in situations where light curing is impractical, such as in deep cavities or bulk restorations.
- Strong, Durable Restorations:
- The polymerization process creates a strong, cross-linked network of polymer chains, ensuring the restoration is durable and long-lasting.
- Prevents premature curing:
- The stability of the initiators and accelerators in the dark ensures the composite doesn’t cure prematurely, allowing dentists to work with the material without time pressure.
Activation/Initiation System in Dental Composites
The activation/initiation system is the process that starts the polymerization (hardening) of dental composites. This system relies on the generation of free radicals, which are highly reactive molecules that kickstart the chemical reaction needed to transform the soft, moldable composite into a hard, durable restoration. Both monomethacrylate and dimethacrylate monomers (the building blocks of dental composites) polymerize through a mechanism called addition polymerization, which is initiated by free radicals.
How Free Radicals Are Generated
Free radicals can be generated in two main ways:
Chemical Activation:
- In this method, free radicals are produced through a chemical reaction between two components:
- An organic peroxide (e.g., benzoyl peroxide) in one paste.
- An organic amine (e.g., dimethyl-p-toluidine) in another paste.
- When these two pastes are mixed, the amine reacts with the peroxide to generate free radicals.
- These free radicals then attack the carbon double bonds (C=C) in the methacrylate monomers, initiating the polymerization process.
- This method is often used in self-curing composites, where light activation is not practical.
- In this method, free radicals are produced through a chemical reaction between two components:
External Energy Activation:
- Free radicals can also be generated using external energy sources, such as:
- Light: The most common method in dentistry. A blue light (around 465 nm) activates a photosensitizer (e.g., camphorquinone) in the composite, which then generates free radicals.
- Heat: Elevated temperatures can break chemical bonds and produce free radicals, though this method is less common in dental applications.
- Microwave Energy: Rarely used in dentistry, but microwaves can also generate free radicals by exciting molecules in the composite.
- Free radicals can also be generated using external energy sources, such as:
The Polymerization Process
- Initiation:
- Free radicals are generated through chemical activation or external energy activation.
- These free radicals attack the carbon double bonds (C=C) in the methacrylate monomers, creating new reactive sites.
- Propagation:
- The reactive sites on the monomers link together, forming long chains of polymer molecules.
- This process continues as more monomers are added to the growing chains.
- Termination:
- The polymerization reaction stops when the free radicals are consumed or when the chains combine with each other, forming a solid, cross-linked network.
Classifications of Filled Resins
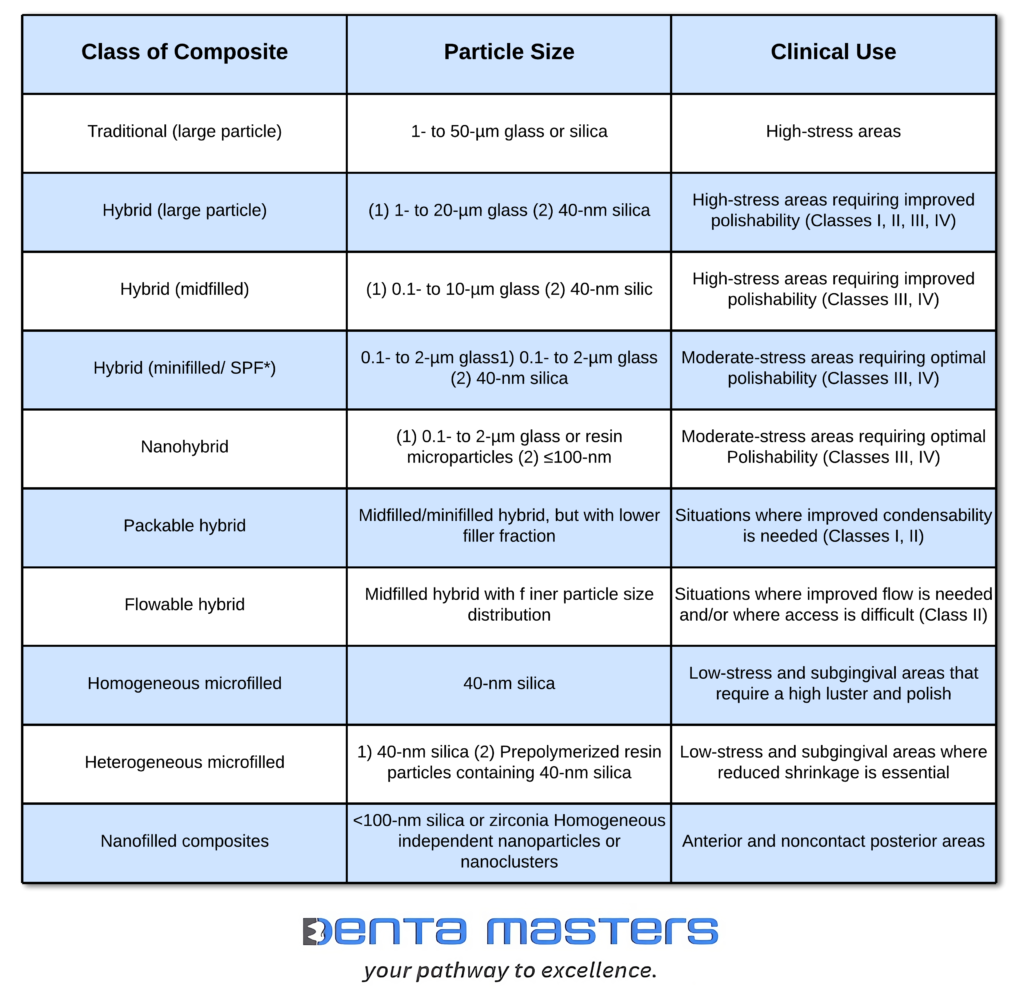
Macrofilled Composites
Macrofilled composites were the first generation of dental composites, developed in the 1960s. While they marked a significant advancement in restorative materials, they have several limitations that led to the development of newer composite types. Here’s an overview of macrofilled composites:
Key Characteristics of Macrofilled Composites
- Filler Material:
- Quartz particles with sizes ranging from 10 to 25 μm.
- Filler Content:
- 70% to 80% by weight.
- Due to the higher density of the filler phase, the volume percentage is typically 10% to 15% lower than the weight percentage.
- Surface Texture:
- The large filler particles result in a rough surface that feels gritty to a dental explorer and can appear rough to the naked eye.
- Aesthetic and Functional Issues:
- Plaque Accumulation: The rough surface increases the likelihood of plaque buildup.
- Staining: The composite can turn slightly gray when rubbed with an instrument, affecting aesthetics.
Clinical Limitations
- Postoperative Sensitivity:
- Macrofilled composites were used before the development of dental bonding systems, leading to poor adhesion and postoperative sensitivity.
- Leakage and Recurrent Decay:
- The lack of effective bonding systems caused microleakage at the restoration margins, increasing the risk of recurrent decay.
- Abrasive Wear:
- The large filler particles made the material abrasive to opposing teeth and restorations.
Why Macrofilled Composites Were Phased Out
- Poor aesthetics: rough surfaces and staining made them unsuitable for visible areas.
- Functional Issues: The high risk of sensitivity, leakage, and recurrent decay limited their use in posterior teeth.
- Advancements in Technology: The development of microfilled, hybrid, and nanofilled composites addressed these limitations, offering smoother surfaces, better aesthetics, and improved durability.
Small (Fine) Particle Composites: A Step Forward in Dental Restoratives
Small-particle composites represent an improvement over macrofilled composites, offering better polishability, aesthetics, and strength. Here’s a detailed look at their characteristics and clinical applications:
Key Characteristics:
- Filler Particle Size:
- Mean particle diameters range from 0.1 to 10 µm (mini-filler and midi-filler).
- Filler Loading:
- 77% to 88% by weight, which is as high or higher than macrofilled composites.
- Provides high hardness and strength but also increases brittleness.
- Polishability:
- More polishable than macrofilled composites (10 to 100 µm), but still cannot achieve a high gloss finish.
- Aesthetics and Durability:
- Offers an excellent balance between polishability, appearance, and durability.
Clinical Applications
- Anterior Restorations: Ideal for Class III, Class IV, and Class V restorations where aesthetics and strength are important.
- General Use: Suitable for areas requiring a balance of aesthetics and functionality.
Advantages Over Macrofilled Composites
- Improved Polishability: Smoother surface compared to macrofilled composites.
- Better Aesthetics: More natural appearance due to smaller particle size.
- High Strength: Comparable or higher filler loading provides excellent hardness and durability.
Limitations
- Brittleness: High filler loading increases brittleness, making the material less suitable for high-stress areas.
- Limited Gloss: Cannot achieve the high gloss finish of microfilled or nanofilled composites.
Microfilled Composites: Aesthetic Excellence with Functional Limitations
Microfilled composites are renowned for their high polishability and aesthetic appeal, making them ideal for specific clinical applications. However, they also have notable limitations, particularly in stress-bearing areas. Here’s a detailed breakdown:
Key Characteristics
- Filler Composition:
- Colloidal silica particles (0.01 to 0.1 µm) embedded in resin filler particles (5 to 50 µm).
- Produced by burning compounds like SiCl4 in an oxygen/hydrogen atmosphere, forming amorphous silica (noncrystalline SiO2).
- Polishability and aesthetics:
- Extremely small filler particles allow for a highly polished surface and excellent translucency, overcoming the surface roughness and opacity of traditional composites.
- Filler Loading:
- 40% to 80% by volume is resin, resulting in:
- Higher water sorption.
- Greater thermal expansion.
- Lower elastic modulus and tensile strength.
- 40% to 80% by volume is resin, resulting in:
Clinical Applications
- Low-Stress Areas: Recommended for Class III and Class V restorations where aesthetics and polishability are critical.
- Non-Stress-Bearing Surfaces: Not suitable for areas subjected to high chewing forces.
Advantages
- Superior Aesthetics: Achieves a high gloss finish and natural appearance.
- Excellent polishability: smoother surface compared to small-particle and macrofilled composites.
- Translucency: Mimics natural tooth structure better than traditional composites.
Limitations
- Weak Bonding: The bond between prepolymerized particles and the clinically cured resin matrix is relatively weak, leading to:
- Wear by Chipping: Microfilled composites are prone to chipping under stress.
- Reduced Tensile Strength: Lower resistance to pulling forces.
- High Water Sorption: Absorbs more water, which can:
- Soften the resin.
- Increase the risk of staining and abrasive wear.
- Thermal Expansion: Higher thermal expansion coefficient compared to tooth structure, leading to:
- Marginal Gaps: Increased risk of microleakage and recurrent decay.
- Unsuitability for Stress-Bearing Areas:
- Wear-Prone Areas: Break down faster in high-stress or wear-prone areas, making them unsuitable for posterior restorations or occlusal surfaces.
Hybrid Composites: Balancing Strength and Aesthetics
Hybrid composites are a versatile class of dental materials designed to combine the strength of small-particle composites with the aesthetic appeal of microfilled composites. Here’s a detailed look at their composition, benefits, and clinical applications:
Key Characteristics
- Filler Composition:
- Mixed Filler System: Combines microfine particles (0.01 to 0.1 µm) and fine particles (0.1 to 10 µm).
- The inclusion of microfine particles improves surface smoothness and polishability, while fine particles enhance strength and durability.
- Filler Loading:
- Slightly lower overall filler loading compared to small-particle composites due to the high surface area of microfine particles, which increases viscosity.
- Polishability and aesthetics:
- It achieves a smoother surface than small-particle composites, though not as glossy as microfilled composites.
- Suitable for restorations where aesthetics are important but strength is also required.
Clinical Applications
- Anterior Restorations:
- Class III and Class IV restorations, where aesthetics and strength are both critical.
- Incisal Edges: Ideal for restoring chipped or worn incisal edges.
- Posterior Restorations:
- Small non-contact occlusal cavities: Suitable for areas with moderate stress.
Advantages
- Balanced Properties: Combines the strength of small-particle composites with the aesthetic appeal of microfilled composites.
- Versatility: Suitable for both anterior and posterior restorations, particularly in areas where aesthetics and moderate strength are required.
- Improved polishability: smoother surface compared to small-particle composites.
Limitations
- Viscosity: The high surface area of microfine particles increases viscosity, making the material slightly more difficult to handle.
- Polishability: While better than small-particle composites, hybrid composites may not achieve the high gloss of microfilled composites.
Nanofilled Composites: The Next Generation of Dental Restoratives
Nanofilled composites represent a significant advancement in dental materials, combining the aesthetic appeal of microfilled composites with improved mechanical properties and handling characteristics. Here’s a detailed look at their unique features and benefits:
Key Characteristics
- Filler Composition:
- Nanoparticles: Sized between 1 to 100 nm, fabricated using advanced methods (not the pyrolytic precipitation process used for colloidal silica).
- Surface Coating: Particles are coated with γ-methacryloxypropyltrimethoxysilane (a silane coupling agent) to prevent agglomeration and reduce viscosity.
- Particle Structure:
- Unlike microfilled composites, where particles form three-dimensional agglomerates, nanofilled composites consist of discrete nanoparticles.
- This structure minimizes viscosity, making the material easier to handle.
- Optical Properties and Polishability:
- Similar to microfilled composites, nanofilled composites offer excellent optical properties and superior polishability, achieving a high gloss finish.
Advantages
- Aesthetic Excellence:
- Mimics natural tooth structure with exceptional translucency and surface smoothness.
- Achieves a high gloss finish, ideal for visible restorations.
- Improved Handling:
- Lower viscosity compared to microfilled composites, making it easier to manipulate and place.
- Enhanced Mechanical Properties:
- Combines the strength of hybrid composites with the aesthetics of microfilled composites.
- Suitable for both anterior and posterior restorations.
- Durability:
- Resistant to wear and chipping, making it a reliable choice for stress-bearing areas.
Clinical Applications
- Anterior Restorations:
- Class III, Class IV, and Class V restorations where aesthetics are critical.
- Veneers and diastema closures.
- Posterior Restorations:
- Class I and Class II restorations in moderate-stress areas.
Classification of Composites by Manipulation
Flowable Composites
Flowable composites are a specialized type of dental filling material designed to be easier to work with in certain situations. They are a modified version of traditional dental composites, with unique properties that make them particularly useful for specific dental procedures.
What Makes Flowable Composites Unique?
- Lower Viscosity (Flowability):
- Flowable composites have a thinner, more liquid-like consistency compared to regular dental fillings. This is achieved by reducing the amount of filler particles in the material.
- Because of this, they can flow easily into tight spaces, spread evenly, and adapt closely to the shape of the cavity. This makes them ideal for creating precise dental anatomy and ensuring a tight seal.
- Ease of Use in Hard-to-Reach Areas:
- Their flowable nature makes them perfect for use in areas that are difficult to access, such as the back teeth (posterior Class II preparations) or deep cavities.
- Dentists often use them to create a well-adapted base or liner in a cavity before placing a stronger restorative material on top.
- Trade-Off in Strength:
- While flowable composites are easy to work with, they are not as strong as traditional composites. The reduced filler content makes them more prone to wear, chipping, and other forms of damage over time.
- Because of this, they are typically used in low-stress areas or as a supportive layer rather than as the main filling material in high-stress areas like chewing surfaces.
What Makes Flowable Composites Unique?
- Lower Viscosity (Flowability):
- Flowable composites have a thinner, more liquid-like consistency compared to regular dental fillings. This is achieved by reducing the amount of filler particles in the material.
- Because of this, they can flow easily into tight spaces, spread evenly, and adapt closely to the shape of the cavity. This makes them ideal for creating precise dental anatomy and ensuring a tight seal.
- Ease of Use in Hard-to-Reach Areas:
- Their flowable nature makes them perfect for use in areas that are difficult to access, such as the back teeth (posterior Class II preparations) or deep cavities.
- Dentists often use them to create a well-adapted base or liner in a cavity before placing a stronger restorative material on top.
- Trade-Off in Strength:
- While flowable composites are easy to work with, they are not as strong as traditional composites. The reduced filler content makes them more prone to wear, chipping, and other forms of damage over time.
- Because of this, they are typically used in low-stress areas or as a supportive layer rather than as the main filling material in high-stress areas like chewing surfaces.
Comparison with Compomers:
Flowable composites are often compared to compomers, which are another type of dental material. Compomers are a hybrid between resin composites and glass ionomers. Like flowable composites, they are easy to use and adapt well to the tooth. However, compomers have an added benefit: they release fluoride, which helps protect the tooth from future decay.
Condensable (Packable) Composites
Condensable composites, also known as packable composites, are a specialized type of dental filling material designed to address some of the limitations of traditional composites. Unlike regular composites, which have a softer, more plastic-like consistency before curing, condensable composites are stiffer and can be packed into a cavity more like dental amalgam. This makes them particularly useful in certain clinical situations.
What Makes Condensable Composites Unique?
- Stiffer, Paste-like Consistency:
- Traditional composites are soft and flowable in their uncured state, making it difficult to pack them tightly into a cavity. Condensable composites, on the other hand, have a thicker, more paste-like consistency.
- This allows dentists to condense (pack) the material vertically and laterally, ensuring it adapts closely to the cavity walls and creates a tight seal.
- Improved handling characteristics:
- The consistency of condensable composites is designed to mimic that of lathe-cut amalgam, a material dentists have long been familiar with. This makes them easier to handle and place, especially in deeper or more complex cavities.
- The material resists flow during placement, which helps maintain the shape and contour of the restoration.
- Enhanced Filler Technology:
- The unique properties of condensable composites come from their filler particles. These composites often include:
- Elongated, fibrous fillers (around 100 µm in length).
- Rough-textured surfaces or branched geometries.
- These fillers interlock with each other, creating a more rigid structure that resists flow and provides better handling during placement.
- The unique properties of condensable composites come from their filler particles. These composites often include:
Why were condensable composites developed?
Traditional composites have a soft, flowable consistency before curing, which can make it challenging to:
- Pack the material tightly into a cavity.
- Ensure intimate contact with the cavity walls.
- Achieve proper contouring and anatomy in the final restoration.
Condensable composites were developed to overcome these challenges by providing a material that
- Can be condensed (packed) like amalgam.
- Maintains its shape during placement.
- Offers better adaptation to cavity walls.
Clinical Uses of Condensable Composites
- Posterior Restorations:
- Condensable composites are particularly useful in posterior teeth (molars and premolars), where the chewing forces are higher and the cavities are often deeper.
- Their packable nature makes them ideal for restoring Class I and Class II cavities.
- Deep Cavities:
- Their ability to be condensed makes them suitable for deep cavities where tight adaptation to the cavity walls is critical.
- Bulk Filling:
- Condensable composites can be placed in larger increments (bulk filling) compared to traditional composites, which often require layering.
Advantages of Condensable Composites
- Better Adaptation: The packable nature ensures intimate contact with cavity walls, reducing the risk of microleakage.
- Ease of Handling: Their consistency is familiar to dentists who have worked with amalgam.
- Improved Contouring: The material holds its shape during placement, making it easier to achieve proper anatomy.
- Suitable for High-Stress Areas: Their stiffness and strength make them more durable in posterior teeth compared to flowable composites.
Limitations of Condensable Composites
- Less Flowable: Their stiffness can make them harder to adapt to very small or intricate cavities.
- Technique Sensitivity: Proper condensation techniques are required to achieve optimal results.
- Not as Versatile as Traditional Composites: They are primarily used for posterior restorations and may not be suitable for all clinical situations.