Cirrhosis is characterized by fibrosis and nodule formation from liver insults and is classified as compensated or decompensated depending on the degree of liver distortion. Compensated cirrhosis patients are asymptomatic and the liver is still able to perform basic functions. Decompensated cirrhosis typically follows compensated cirrhosis. Patients with decompensated cirrhosis often exhibit serious symptoms and complications such as ascites, bacterial infections, variceal bleeding, portal hypertension, or encephalopathy. Prognosis and survival are markedly better in patients with compensated cirrhosis than in those with decompensated disease.5
Since the liver is the primary site of drug metabolism, the disposition and clinical effects of drugs can be altered in patients with cirrhosis. Risk factors for impaired drug effects include altered hepatic blood flow, altered drug pharmacokinetics, reduced drug-binding proteins, and severity of liver dysfunction. Nearly 30% of patients with cirrhosis suffer adverse drug reactions or hepatoxicity if these risk factors are not considered or monitored closely.6 This is because 20% of the drugs in patients with liver cirrhosis are dosed incorrectly.6 Patients with compensated cirrhosis have a lesser extent of impaired drug metabolism compared with patients with decompensated cirrhosis. Pharmacists have a responsibility to ensure safe medication use in patients with cirrhosis. This article will provide pharmacists a practical overview of the impact of cirrhosis on medication therapy and considerations to help prevent adverse drug reactions.
Pharmacokinetic Changes
Changes in cytochrome P450 (CYP 450) activity may occur depending on the degree of liver impairment and other comorbidities.7 Typically, CYP 450 enzyme activity is reduced, resulting in decreased drug clearance and, thereby, increased serum drug concentrations. In general, cytochrome enzymes 1A2 and 3A4 have at least a 50% reduction in activity in cirrhosis while 2C, 2A, and 2B are mostly unaltered.8,9 Because medications with a low extraction ratio (e.g., warfarin, phenytoin, carbamazepine, and lorazepam) rely heavily on the metabolic capacity of the liver for intrinsic clearance through CYP 450 enzymes, these medications will be impacted more significantly than medications with a high extraction ratio (e.g., verapamil, morphine, propranolol, and ketamine).10
Albumin is produced and secreted by the liver. In patients with cirrhosis, albumin production is decreased by 60% to 80%, in some cases leading to hypoalbuminemia.11 Albumin may also decrease due to the dilution effect from water and salt retention.12,13 As such, in the setting of hypoalbuminemia, medications with a high protein-binding affinity to albumin (e.g., medications circulating in bound form >90% of the time) will become unbound in the serum, increasing the risk of toxicity.14 Therefore, a dose reduction may be needed for highly protein-bound medications such warfarin, phenytoin, diazepam, fluoxetine, digoxin, and valproic acid.
Optimizing Medications in Cirrhosis
On average, patients with cirrhosis are prescribed between three and 10 medications.15-17 As such, pharmacists can play a role in optimizing medications to prevent drug-drug interactions, adverse side effects, and toxicities. Initially, pharmacists should review the indications and need for any medications. Pharmacists should also not forget to inquire about OTC medications as well as herbal remedies, as these can lead to undesirable effects in patients with cirrhosis as well.
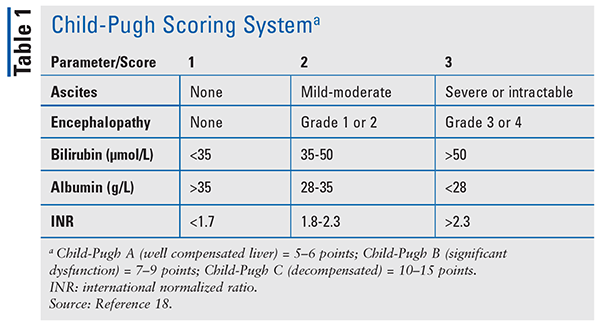
The severity of cirrhosis should be considered prior to making any drug adjustments. While there are a variety of noninvasive indicators and radiologic procedures that can be performed to assess severity, the Child-Pugh score is most commonly used. The Child-Pugh scale was originally developed to evaluate mortality in patients undergoing hepatic resection, but now the scale is used for assessing hepatic impairment for drugs submitted to the FDA for approval. The Child-Pugh score factors in the patient’s bilirubin, albumin, ascites, encephalopathy, and international normalized ratio (INR) and classifies the patient as Class A, B, or C (TABLE 1).18 Another scoring system is the model for end-stage liver disease (MELD) score.19 While MELD was initially created to predict survival in patients with complications of portal hypertension undergoing elective placement of transjugular intrahepatic portosystemic shunts, the major use of the MELD score has been in allocation of organs for liver transplantation.19 The MELD contains three objective variables: INR, creatinine, and total bilirubin.19
While these classification schemes may assist in categorizing the severity of liver disease, they were not designed to reliably estimate the relationship between hepatic impairment and the pharmacokinetics and pharmacodynamics of medications.20 As such, many medication package inserts lack specific information related to drug dosing for hepatic impairment. Additionally, no evidence-based guidelines exist for the use of medications in patients with liver cirrhosis. However, most drugs, even those that are potentially hepatotoxic, can be used safely in patients with cirrhosis as long as the patient is frequently monitored. Considerations in drug use and monitoring recommendations for patients with cirrhosis and common comorbid conditions are provided below.
Hypertension
As hypertensive patients with decompensated cirrhosis develop ascites, their blood pressure can gradually decrease over time. For this reason, these patients should be closely monitored. Because this process typically occurs as a result of the activation of the renin-angiotensin-aldosterone system and subsequent production of endogenous vasoconstrictors, angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs) should be avoided in those with decompensated cirrhosis because of the risk of renal impairment.21 ACEIs and ARBs can be used in patients with compensated cirrhosis; however, blood pressure should be monitored frequently. Diuretic therapy with spironolactone is typically a first-line option for patients with cirrhosis and edema. If an inadequate response is achieved with spironolactone, then furosemide or thiazide diuretics can safely be added to the regimen.22 Calcium channel blockers (CCBs) can be used to regulate blood pressure; however, caution should be taken to avoid the use of CCBs, such as verapamil, without modifying dosages. CCBs should be used at the lowest dose possible because they are cleared primarily by the liver. Reduced hepatic clearance with many antihypertensive medications can result in supratherapeutic drug levels.14
Hyperlipidemia
Due to the risk of toxicity and rhabdomyolosis, statins are typically avoided in patients with decompensated cirrhosis. However, statins that are not extensively metabolized by the liver may be used in patients with compensated cirrhosis if the risk of liver injury is low.23 For example, pravastatin or rosuvastatin can be initiated at low doses and gradually adjusted based on monitoring aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels. Pravastatin and rosuvastatin undergo minimal metabolism through the CYP 450 system prior to excretion. Statins have proven to be beneficial in reducing portal pressures and lowering the risk for hepatocellular carcinoma in patients with compensated cirrhosis.23 Alternatively, cholestyramine may be an option to manage hyperlipidemia in cirrhotic patients.
Diabetes
There are a multitude of options for the treatment of diabetes. Because of varying methods of metabolism, certain antidiabetic agents are preferred over others. Insulin is regarded as the safest and most efficacious treatment of diabetes in patients with cirrhosis regardless of the progression or severity of disease. However, there is an increased risk of hypoglycemia due to the significant amount of malnourishment present in most of these patients. As a result, more frequent monitoring of blood glucose levels is recommended in patients on insulin. Glucagon-like peptide-1 (GLP-1) receptor agonists are not primarily metabolized by the liver, and there is limited evidence to suggest that the levels of these agents are significantly altered in patients with cirrhosis. As a result, GLP-1 agonists can be used without dosage adjustments in patients with compensated cirrhosis or Child-Pugh Class A. While they can also be used cautiously in Child-Pugh Class B, they should not be used in Class C due to a scarcity of evidence to support use in these patients.23 Metformin is considered first-line therapy in patients with type 2 diabetes; however, there is conflicting evidence regarding the use in patients with cirrhosis. Product information and guidelines recommend avoiding metformin in patients with liver disease due to the increased risk of lactic acidosis.24,25 Clinical trials have demonstrated benefits of metformin in patients with stable chronic liver disease at a maximum dose of 1,500 mg/day.26,27 More recently, a study revealed that the pharmacokinetics of metformin are not altered sufficiently in chronic liver disease patients.28
Sulfonylureas should be used with caution in cirrhotic patients. A combination of reduced metabolism by the liver and decreased protein binding to albumin as a result of hypoalbuminemia increases the risk of hypoglycemia related to the use of these agents.27 If used in this patient population, then sulfonylureas with a short half-life, like glipizide or glyburide, are recommended and should be initiated at low doses.27 Pioglitazone should be avoided in patients with decompensated cirrhosis as it can precipitate edema; however, in patients with compensated cirrhosis without edema, pioglitazone can be used with caution.29 Dipeptidyl peptidase-4 (DPP-4) inhibitors can be used cautiously without dosage adjustment in cirrhotic patients; however, they are not preferred in Child-Pugh Class C. Sodium-glucose linked transporter-2 (SGLT-2) inhibitors show mild alterations in drug concentration in cirrhotic patients. Product information suggests that dosage alterations are not necessary in the setting of mild-to-moderate hepatic impairment; however, it is recommended that these agents be used at a lower dose upon initiation.27
Acid Reflux
In patients who have cirrhosis and acid reflux, proton pump inhibitors (PPIs) are commonly prescribed despite the potential increased risk of spontaneous bacterial peritonitis and hepatic encephalopathy associated with PPI use.30 Since the pharmacokinetic profile of PPIs vary, agents that are less affected by cirrhosis are preferred. As such, esomeprazole is favored in cirrhotic patients as it has shown less inhibitory potency compared with lansoprazole, rabeprazole, pantoprazole, and omeprazole.31 When histamine-2-receptor antagonists are indicated, famotidine is the preferred agent since dose adjustments are not needed in the setting of cirrhosis, as it undergoes minimal first-pass metabolism.32,33 However, cimetidine should be avoided due to an increased risk of hepatic encephalopathy.34
Medications to Avoid
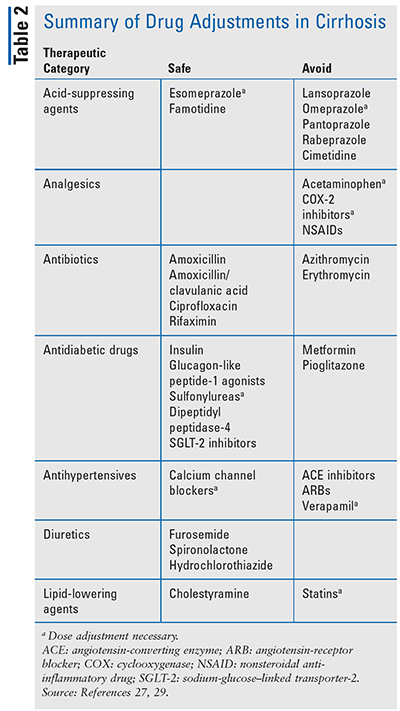
While some medications are deemed safe, some require dose adjustments and frequent monitoring; others should be avoided altogether because of their potential to cause harm in cirrhotic patients (TABLE 2). Acetaminophen should be avoided due to the risk of hepatotoxicity. Patients with alcohol-induced cirrhosis who are taking acetaminophen have an increased risk of worsening liver disease due to the increased production of a toxic metabolite, N-acetyl-p-benzoquinone imine.35 If acetaminophen therapy is necessary, then the maximum daily amount should be less than 2,000 mg. Azithromycin, erythromycin, and clindamycin represent another class of medications that have an increased risk of hepatotoxicity.36 Azithromycin has been shown to have not only an increased risk of acute liver injury but also increased mortality in patients with cirrhosis.36 Methotrexate has been shown to cause hepatotoxicity, possibly due to folate depletion.37 Because these effects can be increased in patients with cirrhosis, methotrexate should be avoided. If the benefit of methotrexate therapy outweighs the risk, frequent monitoring is required.
Other medications that should be avoided include abacavir, COX-2 inhibitors, nonsteroidal anti-inflammatory drugs, direct oral anticoagulant agents, sertraline, and tacrolimus. Herbal supplements have been shown to induce liver injury. Green tea extract is most commonly associated with liver injury; however, the mechanism is unknown.38 The use of herbal supplements is not recommended in cirrhosis patients. It is beyond the scope of this article, as a practical and concise review, to identify all of the many medications that should also be avoided; as such, pharmacists should serve as a key resource for providers and patients in identifying those that should be avoided.
Conclusion
Cirrhosis can have dramatic impacts on drug processing. A clear understanding of how these impacts may affect drug dosing is crucial so that pharmacists can appropriately optimize medications to avoid adverse drug reactions or toxicities. Pharmacists are also in a unique position to influence monitoring for potential adverse events and educate patients with cirrhosis on use of medications.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
REFERENCES
1. CDC. Chronic liver disease and cirrhosis. Updated October 10, 2019. www.cdc.gov/nchs/fastats/liver-disease.htm. Accessed September 15, 2020.
2. Asrani SK, Devarbhavi H, Eaton J, Kamath PS. Burden of liver diseases in the world. J Hepatol. 2019;70(1):151-171.
3. Scaglione S, Kliethermes S, Cao G, et al. The epidemiology of cirrhosis in the United States: a population-based study. J Clin Gastroenterol. 2015;49(8):690-696.
4. Schuppan D, Afdhal NH. Liver cirrhosis. Lancet. 2008;371(9615):838-851.
5. Zipprich A, Garcia-Tsao G, Rogowski S, et al. Prognostic indicators of survival in patients with compensated and decompensated cirrhosis. Liver Int. 2012;32(9):1407-1414.
6. Franz CC, Hildbrand C, Born C, et al. Dose adjustment in patients with liver cirrhosis: impact on adverse drug reactions and hospitalizations. Eur J Clin Pharmacol. 2013;69(8):1565-1573.
7. Dietrich CG, Götze O, Geier A. Molecular changes in hepatic metabolism and transport in cirrhosis and their functional importance. World J Gastroenterol. 2016;22(1):72-88.
8. Fisher CD, Lickteig AJ, Augustine LM, et al. Hepatic cytochrome P450 enzyme alterations in humans with progressive stages of nonalcoholic fatty liver disease. Drug Metab Dispos. 2009;37(10):2087-2094.
9. Elbekai RH, Korashy HM, El-Kadi AO. The effect of liver cirrhosis on the regulation and expression of drug metabolizing enzymes. Curr Drug Metab. 2004;5(2):157-167.
10. Palatini P, De Martin S. Pharmacokinetic drug interactions in liver disease: an update. World J Gastroenterol. 2016;22(3):1260-1278.
11. Garcia-Martinez R, Caraceni P, Bernardi M, et al. Albumin: pathophysiologic basis of its role in the treatment of cirrhosis and its complications. Hepatology. 2013;58(5):1836-1846.
12. John S, Thuluvath PJ. Hyponatremia in cirrhosis: pathophysiology and management. World J Gastroenterol. 2015;21(11):3197-3205.
13. Dudley FJ. Pathophysiology of ascites formation. Gastroenterol Clin North Am. 1992;21(1):215-235.
14. Lewis JH, Stine JG. Review article: prescribing medications in patients with cirrhosis—a practical guide. Aliment Pharmacol Ther. 2013;37(12):1132-1156.
15. Kuo SZ, Haftek M, Lai JC. Factors associated with medication non-adherence in patients with end-stage liver disease. Dig Dis Sci. 2017;62(2):543-549.
16. Polis S, Zang L, Mainali B, et al. Factors associated with medication adherence in patients living with cirrhosis. J Clin Nurs. 2016;25(1-2):204-212.
17. Volk ML, Tocco RS, Bazick J, et al. Hospital readmissions among patients with decompensated cirrhosis. Am J Gastroenterol. 2012;107(2):247-252.
18. Tsoris A, Marlar CA. Use of the Child Pugh score in liver disease. May 17, 2020. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; January 2020. www.ncbi.nlm.nih.gov/books/NBK542308/.
19. Kamath PS, Kim WR. Advanced Liver Disease Study Group. The model for end-stage liver disease (MELD). Hepatology. 2007;45(3):797-805.
20. Talal AH, Venuto CS, Younis I. Assessment of hepatic impairment and implications for pharmacokinetics of substance use treatment. Clin Pharmacol Drug Dev. 2017;6(2):206-212.
21. Runyon BA; AASLD. Introduction to the revised American Association for the Study of Liver Diseases Practice Guideline management of adult patients with ascites due to cirrhosis 2012. Hepatology. 2013;57(4):1651-1653.
22. Qavi AH, Kamal R, Schrier RW. Clinical use of diuretics in heart failure, cirrhosis, and nephrotic syndrome. Int J Nephrol. 2015;2015:975934.
23. Wright AP, Adusumalli S, Corey KE. Statin therapy in patients with cirrhosis. Frontline Gastroenterol. 2015;6(4):255-261.
24. Glucophage package insert. Princeton, NJ; Bristol-Myers Squibb Company; 2017.
25. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2012;35(6):1364-1379. (Published correction appears in Diabetes Care. 2013;36(2):490).
26. Crowley MJ, Diamantidis CJ, McDuffie JR, et al. Clinical outcomes of metformin use in populations with chronic kidney disease, congestive heart failure, or chronic liver disease: a systematic review. Ann Intern Med. 2017;166:191-200.
27. Gangopadhyay KK, Singh P. Consensus statement on dose modifications of antidiabetic agents in patients with hepatic impairment. Indian J Endocrinol Metab. 2017;21(2):341-354.
28. Smith FC, Stocker SL, Danta M, et al. The safety and pharmacokinetics of metformin in patients with chronic liver disease. Aliment Pharmacol Ther. 2020;51(5):565-575.
29. Weersink RA, Bouma M, Burger DM, et al. Evidence-based recommendations to improve the safe use of drugs in patients with liver cirrhosis. Drug Saf. 2018;41(6):603-613.
30. Weersink RA, Bouma M, Burger DM, et al. Safe use of proton pump inhibitors in patients with cirrhosis. Br J Clin Pharmacol. 2018;84(8):1806-1820.
31. Li XQ, Andersson TB, Ahlström M, Weidolf L. Comparison of inhibitory effects of the proton pump-inhibiting drugs omeprazole, esomeprazole, lansoprazole, pantoprazole, and rabeprazole on human cytochrome P450 activities. Drug Metab Dispos. 2004;32(8):821-827.
32. Ohnishi K. Effects of hepatic disease on the pharmacokinetics of famotidine and effects of famotidine on hepatic hemodynamics and peptic ulcer. Hepatogastroenterology. 1990;37 Suppl 1:6-10.
33. Famotidine package insert. Bridgewater, NJ: Valeant Pharmaceuticals North America LLC; 2018.
34. Levine ML. Cimetidine-induced coma in cirrhosis of the liver. JAMA. 1978;240(12):1238.
35. Chandok N, Watt KD. Pain management in the cirrhotic patient: the clinical challenge. Mayo Clin Proc. 2010;85(5):451-458.
36. Martinez MA, Vuppalanchi R, Fontana RJ, et al. Clinical and histologic features of azithromycin-induced liver injury. Clin Gastroenterol Hepatol. 2015;13(2):369-376.e3.
37. Conway R, Carey JJ. Risk of liver disease in methotrexate treated patients. World J Hepatol. 2017;9(26):1092-1100.
38. Navarro VJ, Khan I, Björnsson E, et al. Liver injury from herbal and dietary supplements. Hepatology. 2017;65(1):363-373.